Kim Kardashian West warning letter from FDA criticised by Sudler's SVP group creative director
The Food & Drug Association's (FDA) warning to Kim Kardashian West over her endorsement of a morning sickness drug via social media shows the "need to reassess" its guidelines claims Sudler's SVP group creative director, Chris Duffey.
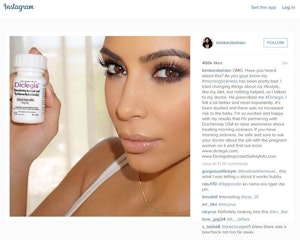

Speaking to The Drum after Kardashian West received a warning letter from the association asking her to remove her Instagram post endorsing Diclegis, Duffey was critical of the move.
“New draft guidance from the FDA on Interactive Promotional Media specifically through social media, just came out in early 2014," he stared. "This warning letter signals a need to reassess. We need to move beyond just 'reminder promotion' and truly have the opportunity to have an open and informed modern day dialogue about health in the social sphere – there is 'one click to safety' legislation in progress that would have made this legal and compliant.”
Duffey added that he felt the letter had "big implications" within the health sector which he said already had "too skittish" rules around social media
Kardashian West claimed her doctor had prescribed the drug, but has removed the post since receiving the letter which said that she failed to reveal the associated risk information relating to Diclegis.
Sudler is a healthcare creative marketing agency and part of the WPP Network.

